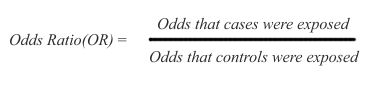Scientific Figures
Study types in epidemiology
Retrospective cohort (or longitudinal) studies
- These studies record different individual recalls of disease incidence in groups of people possibly exposed to PS to varying degrees during the previous course of their lifetimes. In such studies, risk is estimated from differences of incidence in relation to differences in PS exposure. Only a handful of such studies have been performed in regard to PS.
Case-control studies
- These constitute by far the majority of PS studies. They record different individual recalls of possible lifetime PS exposure in two groups of people. One of these groups is composed exclusively of subjects all having the disease under study (lung cancer, for instance): this group is called the cases. The other is composed of subjects who are all free of the disease under study: this group is called the controls.
- In case-control studies the incidence is 0% in the controls and 100% in the cases. Therefore, a key understanding is that in such studies risks are conjectured as differentials of exposure recall, and not actually estimated as differentials of disease incidence. Increased risk is inferred but not directly estimated if exposure is found to be higher among cases, and protection is inferred but not directly estimated if exposure is found to be higher among controls.
Please note that the term “individual recall” means the recollections of individual people concerning the phenomenon that the researchers are interested in. In other words, researchers in these studies use people’s memories as to guess the actual amount of second-hand smoke that they were exposed to, and the comparison of the case and control groups is based on this recollection. Obviously, this fact alone is a considerable “wild card” when it comes to the reliability of the basic data upon which the study depends.
Relative Risk/Odds Ratio
The arithmetic of risk calculation is the same for cohort and case-control studies, except for a difference in terminology. In cohort studies the ratio used for risk calculation is called RR (relative risk), while in case control studies the ratio is called OR (odds ratio).
However, the important difference is that the cohort studies estimate risk directly as differentials of disease incidence, since cohort studies observe only the health outcomes that could be associated with exposure to a particular factor. The case-control studies only assume risk from differentials of exposure, since they are designed to observe the exposure that subjects may have had in the past to the factor of interest.
Let us go into a bit more detail as to how the calculations are done – but don’t worry, it is simple arithmetic!
In cohort studies, risk is measured as a difference in disease incidence between exposed and non-exposed subjects. The risk is defined as relative risk (RR), and it is expressed this way:
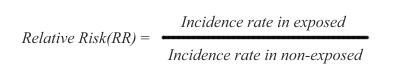
Thus, the disease incidence rate in the exposed subjects is simply divided by the incidence rate in non-exposed subjects. The RR ratio reflects that a certain incidence of disease is observed in both non-exposed and exposed subjects, due to multiple background causes operating in conjunction with, or entirely separate from the exposure under study. Therefore, risk in the exposed is said to be an increment or decrement of incidence, relative to the basic incidence of the non-exposed subjects.
In the RR ratio above, if the rates are the same in exposed and non-exposed subjects, the RR=1 and therefore there is no risk differential. If RR is greater than 1, the risk is said to be increased in the exposed subjects. If RR is smaller than 1, the risk is said to be decreased in the exposed subjects, indicating that the exposure under study might be possibly protective.
Because case-control studies infer but do not directly estimate possible risk, their results are expressed as odds ratios (OR), namely the ratio between the odds (expressed as % or other rate) of being exposed for the cases and the controls:
In the above ratio, if the odds are the same in exposed and non-exposed subjects, the OR=1 and there is no inference of difference in risk. If OR is greater than 1, there is an inference of increased risk in the cases. If OR is smaller than 1, there is an inference of decreased risk for the cases, presuming that the exposure may possibly protect for the disease under study.
Both cohort and case-control studies are affected by similar difficulties of design, data collection, and interpretation — difficulties that are far worse for case-control studies that uniquely rely on vague recollections of exposure.
How to interpret scientific epidemiology reports?
"In epidemiologic research, [increases in risk of less than 100 percent] are considered small and are usually difficult to interpret. Such increases may be due to chance, statistical bias, or the effects of confounding factors that are sometimes not evident". Source: National Cancer Institute, Press Release, October 26, 1994
"As a general rule of thumb, we are looking for a relative risk of 3 or more before accepting a paper for publication." - Marcia Angell, editor of the New England Journal of Medicine
"My basic rule is if the relative risk isn't at least 3 or 4, forget it." - Robert Temple, director of drug evaluation at the Food and Drug Administration.
"An association is generally considered weak if the odds ratio [relative risk] is under 3.0 and particularly when it is under 2.0, as is the case in the relationship of ETS and lung cancer." - Dr. Kabat, IAQC epidemiologist